Background and current use
Leishmaniasis caused by Leishmania parasites and transmitted through the bite of sand flies is affecting humans and animals mainly dogs in the Mediterranean basin including Southern European countries. Climate change has led to the creation of new ecological niches favourable to the establishment of new invasive vectors species including sand flies. The geographical distribution of sand flies is spreading north toward Northern European countries by colonizing new areas with favourable ecological conditions. Through the CLIMOS (Climate Monitoring and Decision Support Framework for Sand Fly-Borne diseases Detection and Mitigation) project, we aimed to understand the transmission dynamics of leishmaniasis and diseases caused by sand fly-borne arbovirus such as Toscana virus in order to develop a new approaches for controlling sand flies. A comprehensive understanding of the ecology and the biology of sand fly vectors is essential for an effective control of sand flies and sand fly-borne diseases. Monitoring of sand flies is a cornerstone in understanding the ecology and the biology of sand fly vectors.
While larvae, nymphs, and eggs are difficult to find in their natural environment, monitoring of sand flies is limited to the adult stage. Entomological survey is of major ecological importance because it allows the determination of sand fly fauna, the densities, the seasonal activities and the infection prevalence in potentially emerging foci of leishmaniasis. For the monitoring of sand flies, two major types of traps are used: interceptive such as sticky traps (Chelbi et al., 2007) or attractant-based traps such as CDC light traps (Chelbi et al., 2008).
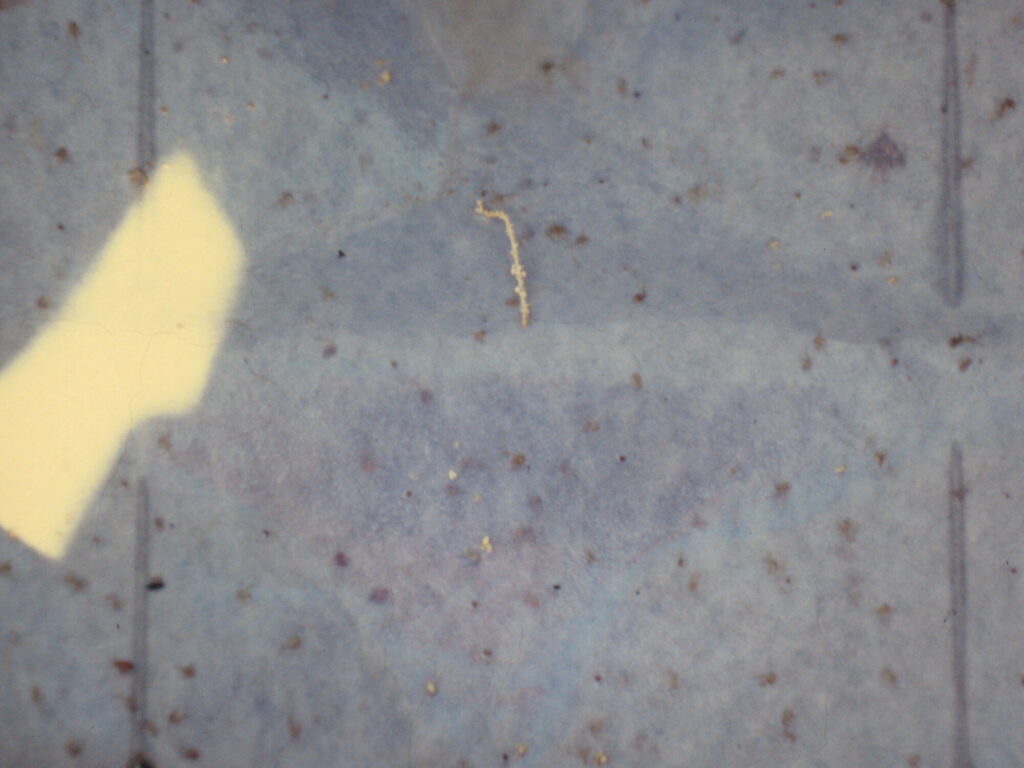
Sampling of sand flies consists of using white papers previously impregnated with castor oil for at least 2-3 days in order to become more transparent, adhesive, and sticky. Thirteen sticky traps of 20×20 cm (total surface is 1 m2) are attached with one cord and are spaced evenly 2 m above the ground in bedrooms or animal shelters (Figure 1) or placed individually at the entry of rodent’s burrows or rock crevices (Figure 2). One trapping unit (13 sticky papers) is placed every week or two for two to three consecutive nights during the sand fly season (Figure 1). Collected sand flies as shown in Figure 3 are easily removed with painting brush from sticky traps, identified to species level using identification keys, and tested for the presence of Leishmania parasites either by microscopy following dissection or by PCR. For sand-fly borne arbovirus, collected sand flies are analyzed by PCR.
Arguments in favour of sticky traps for sand fly monitoring
White sheet papers previously impregnated with castor oil for at least 2-3 days become more transparent, adhesive, and sticky, allow an easily removal of attached sand flies. Compared to other oils such as sesame oil (El Naiem et al., 2020), castor oil is odourless, highly viscous, technical grade, and available worldwide. Commercial glues have a strong odour and may be repulsive. In addition, it precludes the removal of collected sand flies. Sticky traps are widely used for sand fly monitoring and they are considered as environmentally friendly with limited impact on non-target species such as pollinators. Compared to other sampling method such as the miniature CDC light traps, stick traps are not lured with an attractant such as light, CO2, or sugar, and subsequently, this method is not selective providing a realistic image about circulating sand fly species in a given area. Compared to animal bait traps, and White Shannon traps, it was shown that sticky traps, and light traps baited with Carbone dioxide, are the most effective in collecting sand flies (Arzamani et al., 2019). In addition to their effectiveness, this method is cheap, easy to set up and do not rely on power source for activation particularly when filed sites are far away from laboratories. Sticky traps can be setup in bedrooms at night because they are noiseless. Sticky traps are not selective allowing: 1) the collection of a low number of specimens to be identified rapidly, and 2) the determination of the density (defined as the total number of a sand fly species per m²) which represents a more appropriate unit of measuring indexes of biodiversity from an ecological perspective.
CDC light traps are attractive only to sand fly species with positive phototactism and collect a significant number of these specimens. To determine the abundance of a sand fly species, it is required to identify individually a high number of specimens which is extremely time consuming. CDC light traps are noisy particularly when placed in bedroom during bedtimes. CDC traps may fail during operation.
Criticisms and concerns
It is of major importance to point that sticky traps should be used by a well-trained medical entomologist with a good experience in field ecology in order to not skewing the monitoring of sand flies. A major drawback is the dust precluding the adhesiveness of sticky traps toward sand flies mainly when they are used outside and subsequently, they need to be replaced every night. Sticky traps can be easily removed by wind particularly when placed outside.
Improvements
Sticky traps efficiency needs to be enhanced. To maximise sand fly collection from different biotopes including intra-domiciliary, animal shelters, or rodent’s burrows, studies using different colours of sticky traps other than white sheet are needed. To fine tune sand fly collection by sticky traps, studies are needed to adjust their height and orientation as they may differ according to sand fly species (Müller et al., 2015; Elnaiem et al., 2020; Yousefi et al., 2025).
As rodent are major reservoir host for Leishmania parasites, using rolled-up sticky traps placed at the entry of rodent’s burrow (Fig. 4) act as disturbing the movement of rodents and therefore, the latter tends to take them inside the burrows or chew them (Fig. 5). Knowing the behaviour of sandflies, their flight is jerky, and with this method most of them can’t be cached by the rolled sticky trap.
However, flat sticky traps (Fig. 2, 3) placed at the entrance of burrows are significantly less removed and/or chewed by rodents (Fig. 6). This method allows to collect sandflies coming out from the rodent’s burrow and those going inside (Fig. 3) and subsequently could be more efficient.
Potential use of sticky traps in an IPM program
Male sex pheromones of Lutzomyia longipalpis attract females. The analogue of the natural sex pheromone has been synthetized: (S)-9-methylgermacrene-B, and its analogue (+)-9-methylgermacrene (Hamilton, 2008). A long-lasting lure, which releases the synthetic male sex pheromone in chicken shed is attractive to both sexes of L. longipalpis for up to 12 weeks under field conditions and therefore, these lures are suitable for sand fly control (Bray et al., 2014).
Sticky traps has the potential to be used in combination with specific sand fly pheromone-based lure to attract and kill both sex. Thus, this approach may exert a long term negative pressure on population fecundity. In addition, this approach is of highly significance as it does not rely on the use of chemical insecticide and can be used in different settings such as intra-domiciliary and/or peri-domiciliary biotopes. Sand fly control based on using sticky traps in combination with pheromone-based lure in an integrated vector management deserves further investigations.
Conclusion
Sticky traps are useful tools for monitoring sand flies in a given areas. In addition, sticky traps have a great potential to be used in combination with pheromone-based lure for controlling sand fly populations through an attract-and-kill strategy.
References
- Arzamani, K., Rassi, Y., Vatandoost, H., Akhavan, A.A., Abai, M.R., Alavinia, M., Akbarzadeh, K., Mohebali, M., Rafizadeh, S., Karimian, F., Badakhshan, M., Absavaran, A. 2019. Comparative performance of different traps for collection of phlebotominae sand flies and estimation of biodiversity indices in three endemic leishmaniasis foci in north Khorasan province, northeast of Iran. Arthropod-Borne Dis. 13: 399–406
- Bray DP, Carter V, Alves GB, Brazil RP, Bandi KK, et al. (2014) Synthetic Sex Pheromone in a Long-Lasting Lure Attracts the Visceral Leishmaniasis Vector, Lutzomyia longipalpis, for up to 12 Weeks in Brazil. PLoS Negl Trop Dis 8(3): e2723. doi:10.1371/journal.pntd.0002723
- Chelbi, I., Zhioua, E. 2007. Phenology of Phlebotomus papatasi Scopoli (Diptera: Psychodidae) relative to seasonal variation of the prevalence of zoonotic cutaneous leishmaniasis in Central Tunisia. Journal of Medical Entomology, 44: 385-388.
- Chelbi, I., Kaabi, B., Derbali, M., Ben Hadj Ahmed, S., Dellagi, K., Zhioua, E. 2008. Zooprophylaxis: Impact of breeding rabbits in man-made underground holes around houses on reducing the indoor density of Phlebotomus papatasi, principal vector of Leishmania major, etiologic agent of zoonotic cutaneous leishmaniasis in the Old World. Vector-Borne and Zoonotic Diseases, 8: 741-747.
- Elnaiem, D. E., Khogali, A., Alsharif, B., Dakein, O., Jibreel, T., Hassan, M., Edries, H. H., Elhadi,
- H., Elnur, B., Osman, O. F., Boer, M. D., Alvar, J. & Khalid, N. M. 2020. Understanding sand fly sampling methods: sticky traps are attraction-based and not interceptive sampling tools of Phlebotomus orientalis. Parasites & Vectors, 13, 389. https://doi.org/10.1186/s13071-020-04249-1
- Hamilton, J.G.C. 2008. Sandfly pheromone, their biology and potential for use in control programs. Parasite 15: 252-256.
- Müller, G. C., Hogsette, J. A., Kline, D. L., Beier, J. C., Revay, E. E. & Xue, R. D. 2015. Response of the sand fly Phlebotomus papatasi to visual, physical and chemical attraction features in the field. Acta Trop, 141, 32-6.
- Yousefi, S. , Paksa, A., Sanei–Dehkordi, A. , Azizi, K.,Abbasi, M., Shahabi, S., Gheibi, Z., Dabaghmanesh, S., Vahedi, M. ,Danaei, N. , Innovative Trap Approaches for Studying Phlebotomus papatasi: Emphasizing Color and Position, Acta Tropica (2025), doi: https://doi.org/10.1016/j.actatropica.2025.107727